Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Ni
Ni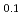 D
D at high
at high 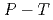 conditions
conditionsShito, Chikara*; Kagi, Hiroyuki*; Kakizawa, Sho*; Aoki, Katsutoshi*; Komatsu, Kazuki*; Iizuka, Riko*; Abe, Jun*; Saito, Hiroyuki*; Sano, Asami; Hattori, Takanori
American Mineralogist, 108(4), p.659 - 666, 2023/04
Times Cited Count:1 Percentile:64.83(Geochemistry & Geophysics)The phase relation and crystal structure of Fe Ni
Ni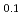 H
H (D
(D ) at high pressures and temperatures up to 12 GPa and 1000 K were clarified by in-situ X-ray and neutron diffraction measurements. Under
) at high pressures and temperatures up to 12 GPa and 1000 K were clarified by in-situ X-ray and neutron diffraction measurements. Under 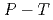 conditions of the present study, no deuterium atoms occupied tetragonal (
conditions of the present study, no deuterium atoms occupied tetragonal ( ) sites of face-centered cubic (fcc) Fe
) sites of face-centered cubic (fcc) Fe Ni
Ni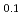 D
D unlike fcc FeH
unlike fcc FeH (D
(D ). The deuterium-induced volume expansion per deuterium
). The deuterium-induced volume expansion per deuterium 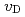 was determined as 2.45(4)
was determined as 2.45(4) 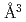 and 3.31(6)
and 3.31(6) 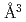 for fcc and hcp phases, respectively, which were significantly larger than the corresponding values for FeD
for fcc and hcp phases, respectively, which were significantly larger than the corresponding values for FeD . The
. The 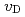 value slightly increased with increasing temperature. This study suggests that only 10% of nickel in iron drastically changes the behaviors of hydrogen in metal. Assuming that
value slightly increased with increasing temperature. This study suggests that only 10% of nickel in iron drastically changes the behaviors of hydrogen in metal. Assuming that 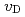 is constant regardless of pressure, the maximum hydrogen content in the Earth's inner core is estimated to be one to two times the amount of hydrogen in the oceans.
is constant regardless of pressure, the maximum hydrogen content in the Earth's inner core is estimated to be one to two times the amount of hydrogen in the oceans.
Ohashi, Tomonori*; Sakamaki, Tatsuya*; Funakoshi, Kenichi*; Hattori, Takanori; Hisano, Naoki*; Abe, Jun*; Suzuki, Akio*
American Mineralogist, 107(3), p.325 - 335, 2022/03
Times Cited Count:1 Percentile:22.72(Geochemistry & Geophysics)The basaltic glass structure were investigated to 18 GPa using in situ X-ray and neutron diffraction. The O-O coordination number (CN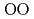 ) starts to rise with maintaining the mean O-O distance (r
) starts to rise with maintaining the mean O-O distance (r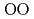 ) above 2-4 GPa, and then CN
) above 2-4 GPa, and then CN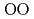 stops increasing and r
stops increasing and r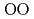 begins to shrink along with the increase in the Al-O coordination number (CN
begins to shrink along with the increase in the Al-O coordination number (CN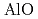 ) above 9 GPa. This is interpreted by the change in the contraction mechanism from tetrahedral network bending to oxygen packing ratio increase via the CN
) above 9 GPa. This is interpreted by the change in the contraction mechanism from tetrahedral network bending to oxygen packing ratio increase via the CN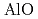 increase. The oxygen packing fraction exceeds the value for dense random packing, suggesting that the oxygen-packing hypothesis cannot account for the pressure-induced structural transformations of silica and silicate glasses. The CN
increase. The oxygen packing fraction exceeds the value for dense random packing, suggesting that the oxygen-packing hypothesis cannot account for the pressure-induced structural transformations of silica and silicate glasses. The CN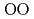 increase at 2-4 GPa reflects the elastic softening of silicate glass, which may causes anomalous elastic moduli of basaltic glass at
increase at 2-4 GPa reflects the elastic softening of silicate glass, which may causes anomalous elastic moduli of basaltic glass at  2 GPa.
2 GPa.
Yuguchi, Takashi*; Yuasa, Haruka*; Izumino, Yuya*; Nakashima, Kazuo*; Sasao, Eiji; Nishiyama, Tadao*
American Mineralogist, 107(3), p.476 - 488, 2022/03
Times Cited Count:2 Percentile:22.72(Geochemistry & Geophysics)The methodology and interpretations in this study provide new insights into the mechanism of myrmekite formation in a granitic system. The presence of micropores in the myrmekites and this study clarified that the genesis of micropores is related to the formation of myrmekites in the Toki granite. The results led to an increased understanding of (1) an estimate of mass transfer between the reactant and product minerals, and the inflow and outflow of components with consideration of the volume change due to micropore formation, (2) the factor controlling the formation of micropores during myrmekitization, and (3) the sequential variations in the hydrothermal fluid chemistry during sub-solidus conditions. The micropores act as a migration pathway for hydrothermal fluid and thus enhance the mass transfer within a granitic body starting with the myrmekite formation and continuing through the present day and into the future.
Yuguchi, Takashi*; Matsuki, Takanobu*; Izumino, Yuya*; Sasao, Eiji; Nishiyama, Tadao*
American Mineralogist, 106(7), p.1128 - 1142, 2021/07
Times Cited Count:4 Percentile:41.95(Geochemistry & Geophysics)This study reveals the hydrothermal alteration processes in a pluton, with a focus on the mass transfer between minerals and hydrothermal fluid. Hydrothermal alteration of the Toki granite in Tono area, central Japan, progressed through the successive processes of chloritization, plagioclase alteration, and precipitation of a carbonate mineral. This paper describes the alteration process of hornblende chloritization, K-feldspar chloritization, and the formation of fracture-filling chlorite through petrography and mineral chemistry. Several types of chloritization reactions (including biotite chloritization) can be characterized by their reaction with the inflow of Al , Fe
, Fe , Mn
, Mn , and Mg
, and Mg and the outflow of H
and the outflow of H SiO
SiO , Ca
, Ca , K
, K +, and F
+, and F . The reactions of chloritization and plagioclase alteration represent the sequential variations in fluid chemistry at temporal conditions from 68 Ma to 51 Ma as the temperature decreased from 350
. The reactions of chloritization and plagioclase alteration represent the sequential variations in fluid chemistry at temporal conditions from 68 Ma to 51 Ma as the temperature decreased from 350 C to 180
C to 180 C.
C.
Yuguchi, Takashi*; Shobuzawa, Kaho*; Ogita, Yasuhiro*; Yagi, Koshi*; Ishibashi, Masayuki; Sasao, Eiji; Nishiyama, Tadao*
American Mineralogist, 104(4), p.536 - 556, 2019/04
Times Cited Count:18 Percentile:72.93(Geochemistry & Geophysics)This study describes the plagioclase alteration process with a focus on the role of micropores, mass transfer and reaction rate in the Toki granitic pluton, central Japan. The plagioclase alteration process involves albitization, K-feldspathization, and the formation of illite, calcite, fluorite and epidote. Such secondary minerals of hydrothermal origin in plagioclase within granitic rocks record the chemical characteristics of the hydrothermal fluid. Our results highlight (1) the nature of micropores such as distribution and volume in plagioclase, (2) the reaction nature of plagioclase alteration inferred by petrography and chemistry, (3) the physical conditions including alteration age and temperature, (4) the sequential variations of the fluid chemistry and (5) the mass transfer rate and reaction rate in the plagioclase alteration.
Yuguchi, Takashi; Sasao, Eiji; Ishibashi, Masayuki; Nishiyama, Tadao*
American Mineralogist, 100(5-6), p.1134 - 1152, 2015/05
Times Cited Count:33 Percentile:72.97(Geochemistry & Geophysics)This paper describes the biotite chloritization process with a focus on mass transfer in the Toki granitic pluton, Central Japan, and also depicts the temporal variations in chemical characteristics of hydrothermal fluid associated with chloritization during the sub-solidus cooling of the pluton. Singular value decomposition (SVD) analysis results in chloritization reaction equations for eight mineral assemblages, leading to the quantitative assessment of mass transfer between the reactant and product minerals, and inflow and outflow of components through the hydrothermal fluid. The matrices for SVD analysis consist of arbitrary combinations of molar volume and closure component in the reactant and product minerals. The eight reactions represent the temporal variations of chemical characteristics of the hydrothermal fluid associated with chloritization: the progress of chloritization results in gradual increase of silicon, potassium and chlorine and gradual decrease of calcium and sodium in the hydrothermal fluid with temperature decrease. The biotite chloritization involves two essential formation processes: Formation Process 1, small volume decrease from biotite to chlorite and large inflow of metallic ions from the hydrothermal fluid, and Formation Process 2, large volume decrease and large outflow of metallic ions into hydrothermal fluid. Chlorite produced during Formation Process 1 dominates over that of Formation Process 2, resulting in the gradual decrease of metallic components in the hydrothermal fluid with chloritization progress. The combination of continuous reactions based on compositional variations in chlorite together with corresponding continuous Al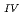 variations gives an indication of the temporal variations in rates of decreasing and increasing concentration of chemical components in the hydrothermal fluid associated with chloritization.
variations gives an indication of the temporal variations in rates of decreasing and increasing concentration of chemical components in the hydrothermal fluid associated with chloritization.
 -bearing Mg-perovskite and its crystal chemistry at high pressure
-bearing Mg-perovskite and its crystal chemistry at high pressureMashimo, Izumi*; Otani, Eiji*; Hirao, Naohisa*; Mitsui, Takaya; Masuda, Ryo*; Seto, Makoto*; Sakai, Takeshi*; Takahashi, Suguru*; Nakano, Satoshi*
American Mineralogist, 99(8-9), p.1555 - 1561, 2014/08
Times Cited Count:7 Percentile:24.02(Geochemistry & Geophysics)Nishida, Keisuke*; Otani, Eiji*; Urakawa, Satoru*; Suzuki, Akio*; Sakamaki, Tatsuya*; Terasaki, Hidenori*; Katayama, Yoshinori
American Mineralogist, 96(5-6), p.864 - 868, 2011/05
Times Cited Count:32 Percentile:69.49(Geochemistry & Geophysics)The density of liquid iron sulfide (FeS) was measured up to 3.8 GPa and 1800 K using the X-ray absorption method. The compression curve of the liquid FeS can be fitted using the Vinet equation of state. Isothermal bulk modulus and its temperature and pressure derivatives were determined by a non-linear least squares fit. Liquid FeS is more compressible than Fe-rich Fe-S liquid.
 -Al(OH)
-Al(OH) ; Neutron diffraction measurements and ab initio calculations
; Neutron diffraction measurements and ab initio calculationsMatsui, Masanori*; Komatsu, Kazuki*; Ikeda, Emi*; Sano, Asami; Goto, Hirotada*; Yagi, Takehiko*
American Mineralogist, 96(5-6), p.854 - 859, 2011/05
Times Cited Count:13 Percentile:38.25(Geochemistry & Geophysics)Neutron powder diffraction analyses of  -Al(OD)
-Al(OD) revealed that the crystals are orthorhombic with space group
revealed that the crystals are orthorhombic with space group  2
2 2
2 2
2 , but not
, but not  as reported previously by X-ray diffraction data. The initial lattice parameters and the atomic positions of both Al and O were taken from previous X-ray structural analyses for the
as reported previously by X-ray diffraction data. The initial lattice parameters and the atomic positions of both Al and O were taken from previous X-ray structural analyses for the  structure, while the H atom positions were determined using ab initio calculations. The
structure, while the H atom positions were determined using ab initio calculations. The  -Al(OH)
-Al(OH) structure possesses one relatively long and two short O-H...O hydrogen bonds. Ab initio calculations are also used to find that
structure possesses one relatively long and two short O-H...O hydrogen bonds. Ab initio calculations are also used to find that  -Al(OH)
-Al(OH) with space group
with space group  2
2 2
2 2
2 transforms to another high pressure polymorph with space group
transforms to another high pressure polymorph with space group  at around 67 GPa, and that the two short hydrogen bonds in
at around 67 GPa, and that the two short hydrogen bonds in  -Al(OH)
-Al(OH) become both symmetric through the transformation, in which the protons are located at the midpoints of the O...O hydrogen bonds.
become both symmetric through the transformation, in which the protons are located at the midpoints of the O...O hydrogen bonds.
Sakamaki, Tatsuya*; Otani, Eiji*; Urakawa, Satoru*; Terasaki, Hidenori*; Katayama, Yoshinori
American Mineralogist, 96(4), p.553 - 557, 2011/04
Times Cited Count:31 Percentile:68.33(Geochemistry & Geophysics)The density of carbonated peridotite magma was measured up to 3.8 GPa and 2100 K using an X-ray absorption method. The bulk modulus of carbonated peridotite magma is larger than that of hydrous peridotite magma. The partial molar volume of CO in magma under high pressure and temperature conditions was calculated. Our results show that the partial molar volume of CO
in magma under high pressure and temperature conditions was calculated. Our results show that the partial molar volume of CO is less compressible than that of H
is less compressible than that of H O, suggesting that, on an equal molar basis, CO
O, suggesting that, on an equal molar basis, CO is more effective than H
is more effective than H O in reducing peridotite melt density at high pressure.
O in reducing peridotite melt density at high pressure.
Sakamaki, Tatsuya*; Otani, Eiji*; Urakawa, Satoru*; Suzuki, Akio*; Katayama, Yoshinori
American Mineralogist, 95(1), p.144 - 147, 2010/01
Times Cited Count:37 Percentile:70.43(Geochemistry & Geophysics)The density of a peridotite magma was measured up to 2.5 GPa and 2300 K using an X-ray absorption method. The method allowed measurement of the density of a peridotite melt under seven different conditions and clarified the pressure and temperature dependence of the density. A fit of the pressure-density-temperature data to the high-temperature Birch-Murnaghan equation of state yielded the isothermal bulk modulus, 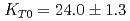 GPa, its pressure derivative,
GPa, its pressure derivative, 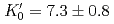 , and the derivative of bulk modulus
, and the derivative of bulk modulus  GPa/K at 2100 K. The large bulk modulus and its pressure derivative of the peridotite melt compared with that of basaltic melt is consistent with previous results from sink-float experiments.
GPa/K at 2100 K. The large bulk modulus and its pressure derivative of the peridotite melt compared with that of basaltic melt is consistent with previous results from sink-float experiments.
Fukushi, Keisuke*; Sato, Tsutomu*; Yanase, Nobuyuki; Minato, Junichi*; Yamada, Hirohisa*
American Mineralogist, 89(11-12), p.1728 - 1734, 2004/11
The sorption mechanism of As(V) on schwertmannite was investigated by both a batch sorption experiment and crystallographic considerations. The batch experiment was carried out as a function of As(V) concentration in acidic solution at 25  C. Crystallographic considerations indicate surface sites of schwertmannite comprise varied surface oxygen (hydroxyl) and SO
C. Crystallographic considerations indicate surface sites of schwertmannite comprise varied surface oxygen (hydroxyl) and SO groups. Sorption experiments showed reactive surface sites for As(V) sorption are surface SO
groups. Sorption experiments showed reactive surface sites for As(V) sorption are surface SO groups. As(V) sorption mechanism involves ligand exchange with solid phase SO
groups. As(V) sorption mechanism involves ligand exchange with solid phase SO . Results also suggest monodentate As(V) coordination with surface adsorbed SO
. Results also suggest monodentate As(V) coordination with surface adsorbed SO sites and bidentate As(V) coordination in structural originated SO
sites and bidentate As(V) coordination in structural originated SO sites. Estimated equilibrium constant for ligand exchange reaction describes the observed As(V) sorption behavior. The surface structure approach in this study reveals reactive surface sites in As(V) sorption on schwertmannite comprise surface SO
sites. Estimated equilibrium constant for ligand exchange reaction describes the observed As(V) sorption behavior. The surface structure approach in this study reveals reactive surface sites in As(V) sorption on schwertmannite comprise surface SO group instead of surface hydroxyl groups identified by former views.
group instead of surface hydroxyl groups identified by former views.
Murakami, Takashi*; Kogure, Toshihiro*; *; Onuki, Toshihiko
American Mineralogist, 83, p.1209 - 1219, 1998/00
no abstracts in English
Murakami, Takashi*; Onuki, Toshihiko; Isobe, Hiroshi; Sato, Tsutomu
American Mineralogist, 82, p.888 - 899, 1997/00
no abstracts in English
Murakami, Takashi; Sato, Tsutomu; Watanabe, Takashi*
American Mineralogist, 78(3), p.465 - 468, 1993/00
no abstracts in English
Murakami, Takashi; B.C.Chakoumakos*; R.C.Ewing*; G.R.Lumpkin*; W.J.Weber*
American Mineralogist, 76, p.1510 - 1532, 1991/00
no abstracts in English